Research
Cytomorphology of BPDCN
In textbook cases, BPDCN cells have nuclei with variable irregularities and fine chromatin. Inconspicuous or one to several small nucleoli are observed. We referred to these cases as “classic BPDCN”.
By analyzing 118 BPDCN cases collected from 52 institutions in Japan, we revealed that MYC alteration (gene rearrangement and overexpression) was recurrent in BPDCN (~40%). It can be prognosed by cytomorphological observation before proper analysis and was associated with drug response (Sakamoto K., Takeuchi K. et al. Leukemia. 2018 Dec;32(12):2590-2603.)
In 2001, we identified a case in which the neoplastic cells morphologically resembled immunoblasts (activated B cells with a round-to-ovoid nucleus and a large centrally located nucleolus). Since then, we have unofficially called such cases “immunoblastoid variant”. In 2014, we encountered an immunoblastoid BPDCN case with 8q24 (MYC locus) rearrangement and MYC expression. Based on this experience, we hypothesized that there was a genotype-phenotype correlation between cytomorphology and 8q24 rearrangement with associated MYC expression in BPDCN.
Association between cytomorphology and MYC status in BPDCN
To investigate this issue, we collected specimens and clinical information from 154 patients from 52 institutions who have been either diagnosed with BPDCN or given an equivalent. Through histopathological review, we confirmed the diagnosis of BPDCN in 118 patients. Cytomorphologically, 62 (53%) were subclassified as classic BPDCN, while 41 (35%) were classified as immunoblastoid BPDCN. Subsequently, we examined the cases for 8q24 rearrangement and MYC expression by FISH and IHC, respectively. Among the 109 cases in which MYC status was evaluable, 41 (38%) and 59 (54%) cases were classified as MYC+BPDCN (both positive by FISH and IHC) and MYC−BPDCN (both negative), respectively. Surprisingly, all evaluable immunoblastoid BPDCN cases were MYC+BPDCN (39/39 cases), whereas 96% (54/56 cases) of the classic BPDCN were MYC–BPDCN, revealing a striking genotype-phenotype correlation between MYC status and cytomorphology in BPDCN (Figure 1).
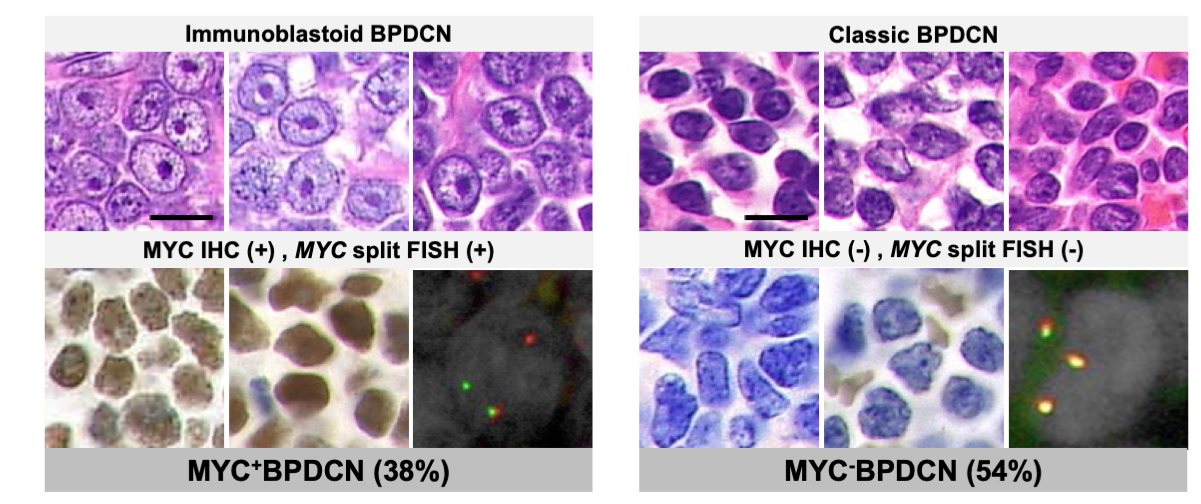
(Based on Sakamoto et al.; Leukemia, 2018)
Clinicopathological comparison between MYC+BPDCN and MYC–BPDCN cases
Statistical analysis of clinicopathological data revealed that MYC+BPDCN patients showed older onset and poorer outcomes than those in MYC–BPDCN patients. Differences in the features of skin lesions were also noted. Most MYC+BPDCN patients developed localized skin tumors, whereas MYC–BPDCN patients tended to exhibit generalized patches/plaques (Figure 2). Although this study was retrospective and needs further validation, these results indicated that several important clinical features also differed depending on the MYC status. The immunophenotype of the two subgroups was also different in some aspects, such as the positive rate of CD56, a BPDCN-characteristic marker. It was 78% in MYC+BPDCN and 98% in MYC–BPDCN. Figure 3 shows an integrative list of 118 BPDCN cases.
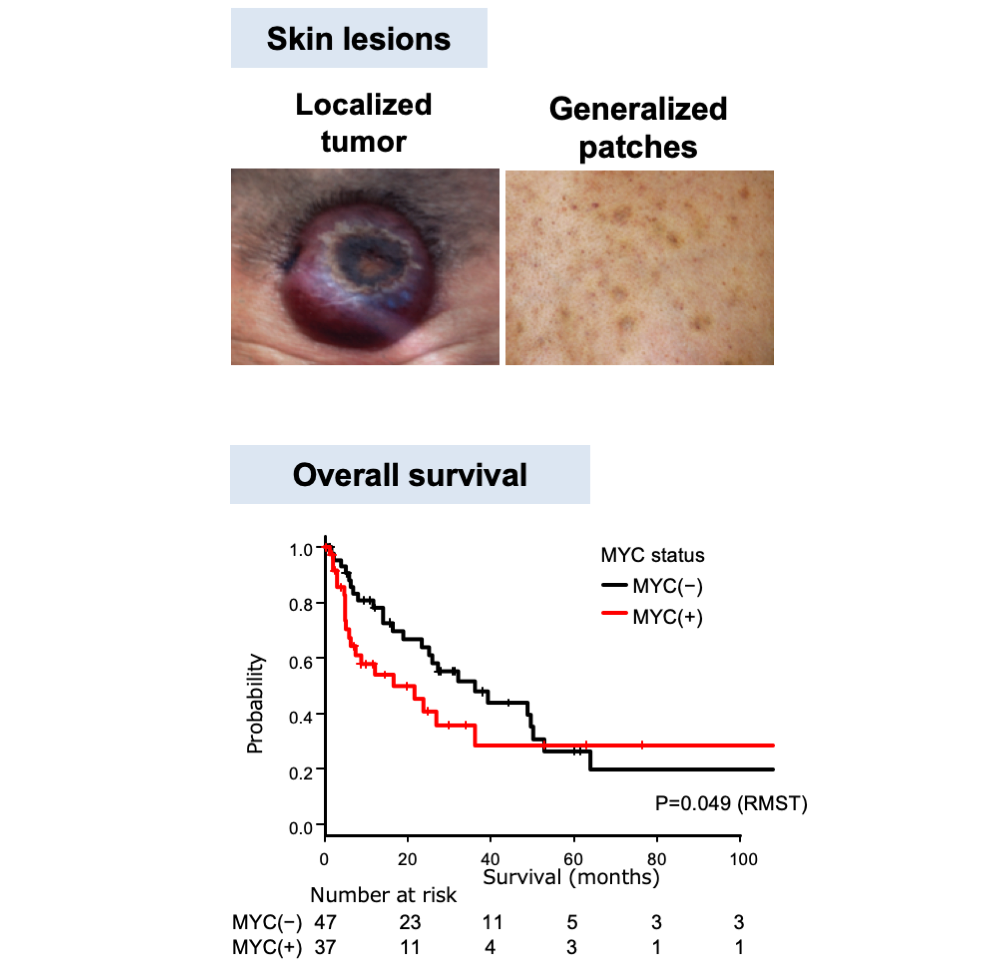
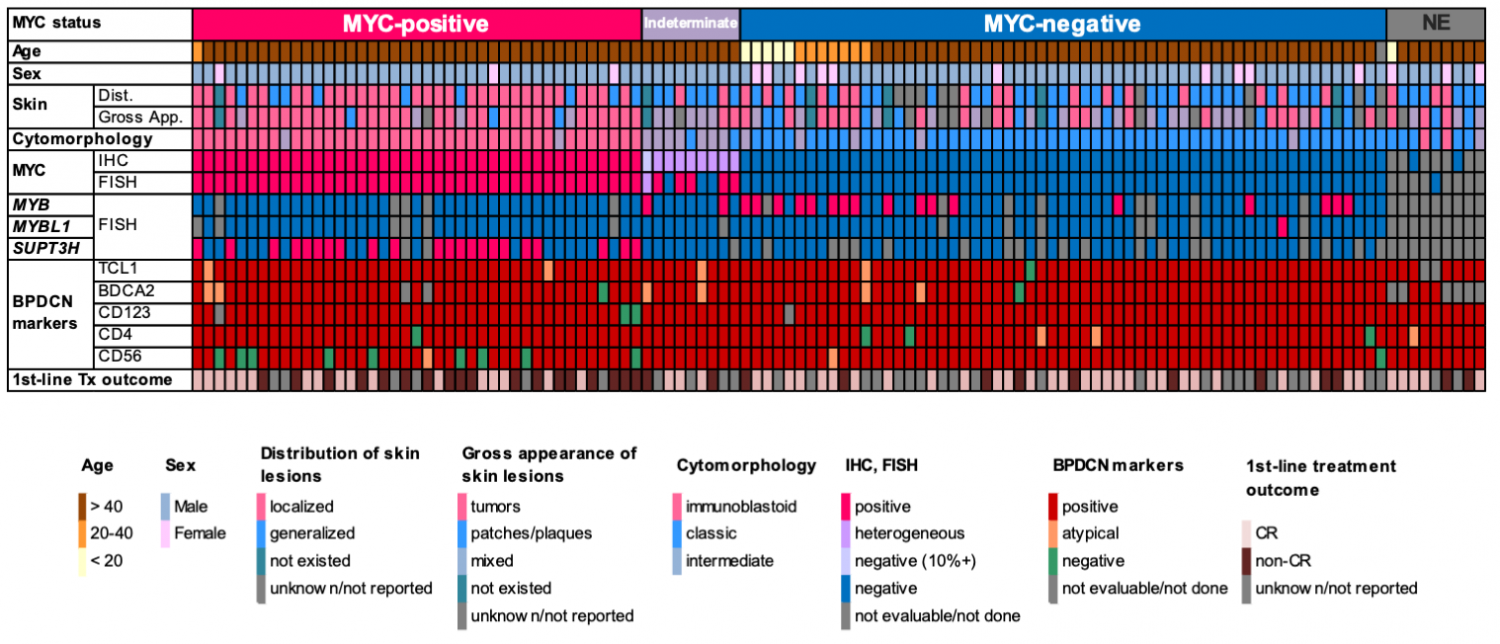
(Adapted form Sakamoto et al.; Leukemia, 2018)
MYC status and drug sensitivity
Among the currently available BPDCN cell lines, we found that CAL-1 and PMDC05 were MYC+BPDCN and MYC–BPDCN, respectively. We used these cell lines to investigate the biological characteristics of MYC+BPDCN and MYC–BPDCN and compared the gene expression profiles of these cell lines. We found MYC as one of the molecules presenting the clearest distinction between the two cell lines. Additionally, MYC knockdown suppressed CAL-1 growth. These results suggest that MYC plays a pivotal role in the growth and maintenance of MYC+BPDCN. Furthermore, bromodomain and extra-terminal protein (BET) inhibitors and aurora kinase inhibitors- the drugs that indirectly suppress MYC- suppressed CAL-1 cell viability more effectively than that of PMDC05 (Figure 4). We further showed that venetoclax, a BCL2 inhibitor, was effective in both CAL-1 and PMDC05, indicating that venetoclax could be used in MYC–BPDCN cases, where BET inhibitors and aurora kinase inhibitors were less effective.
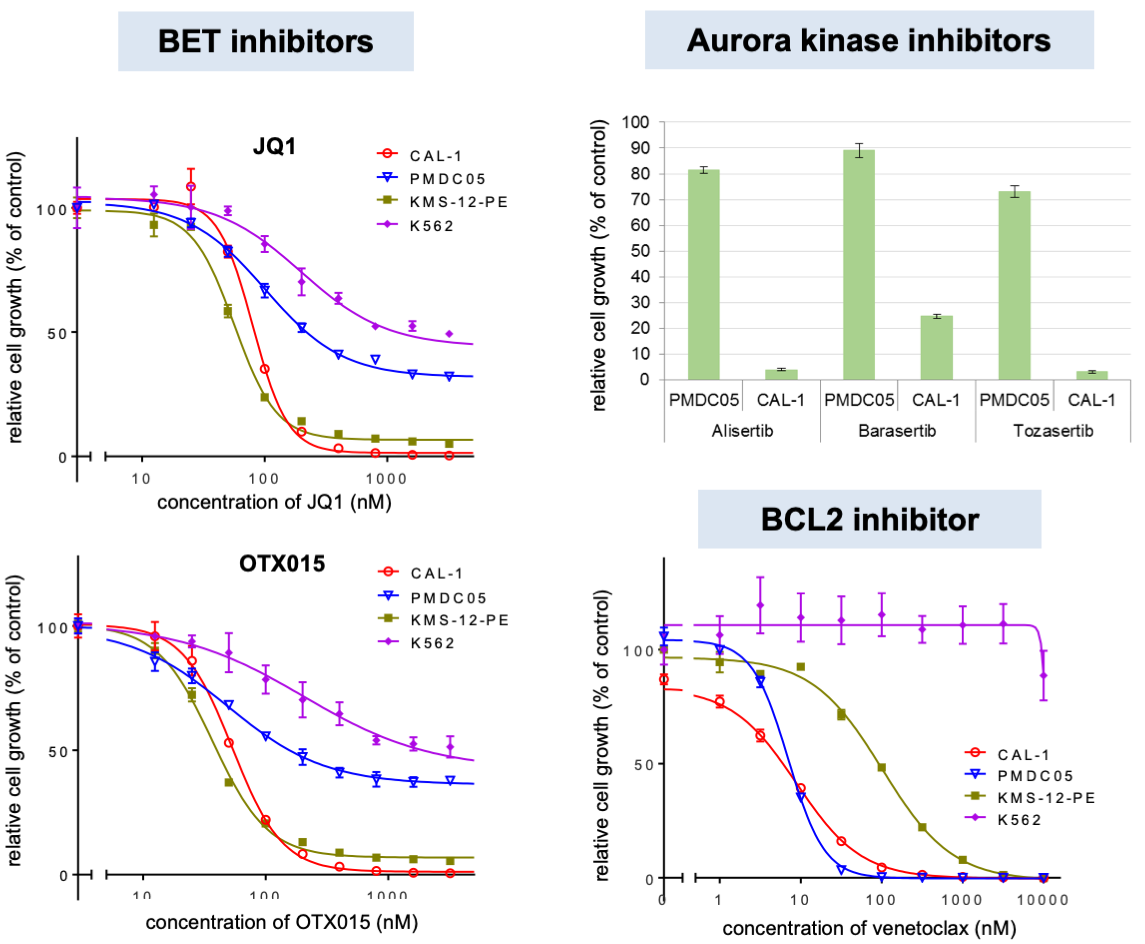
(Based on Sakamoto et al.; Leukemia, 2018)
Future direction
In summary, we revealed that 8q24 rearrangement, MYC expression and immunoblastoid cytomorphology were all strongly associated in BPDCN and might be relevant when choosing a treatment course for BPDCN. Stratification according to MYC status may contribute to the understanding of the pathogenesis of BPDCN and the development of therapeutic strategies for BPDCN. Based on these new insights, we will further advance research to clarify the pathogenesis of this disease.
